
The history of radioactivity began in 1896, when Henri Becquerel stored uranium compounds in a set of photographic plates and observed that there were signs of radiation after the plates were developed. This was followed by two researchers, Marie Curie and G.C. Schmidt, who discovered and published their research into a new compound that emitted radiation signals, just like uranium. They called this compound Thorium (Th), and observed that it was not just an event that happened in isolation, so Marie Curie called this phenomenon “radioactivity”, being a property that some unstable atoms perform as a way of releasing energy so that they can be transformed into more stable atoms, releasing particles during this process, which can be alpha and beta particles or photons.
Months after this discovery, Marie Curie, together with her husband Pierre Curie, made new discoveries, an unknown element that contained 400 times more radioactivity than uranium, so they named it Polonium (Po) after Marie Curie’s country of origin. Then a new compound was presented which contained 900 times more radioactivity than uranium and was named Radium (Ra).
As time went by, the Curie couple and other researchers dedicated years of research to disseminating the knowledge and concepts we have today about radioactivity.
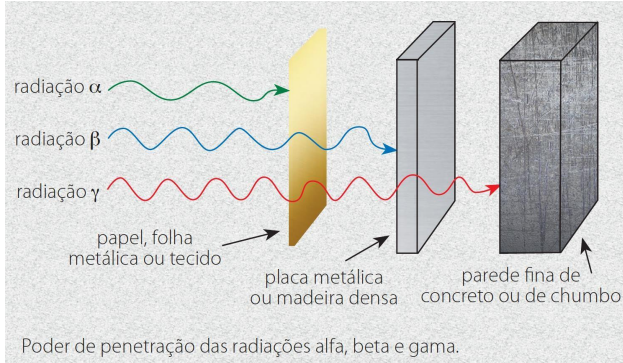
Radiation in its ionic form can be presented in three ways: Alpha (α), Beta (𝛽) and Gamma (γ). Alpha particles (α) are made up of two protons and two neutrons, like the nucleus of a helium atom, so they have the greatest charge and consequently have a larger-scale interaction with other atoms, which in turn will reduce the particle’s energy and thus reduce its penetration capacity. The α particles can be blocked by surfaces such as paper or even fabrics. Beta particles (𝛽) are charged with electrons, contain a smaller charge, but have a greater penetration capacity and can pass through up to 2 cm of living tissue. The speed and damage to health are more pronounced in these particles. Gamma radiation (γ) has no atomic particles and is an electromagnetic wave with high energy, speed and penetrating power, which can cause serious damage to health due to its high penetrating power.
Radioactivity is present in our daily lives, whether it comes from natural or artificial sources, and so we are constantly exposed to it. Therefore, in order to guarantee the protection of human health, various standards and legislation have been implemented with the aim of controlling and monitoring total alpha and total beta radiation in drinking water.
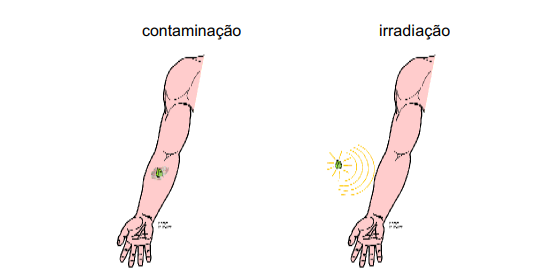
Exposure to radiation can occur in two ways:
Irradiation – This is the process by which exposure occurs without the radioactive source coming into contact with the individual, such as X-ray equipment.
Contamination – Contamination can occur externally, when radioactive material comes into contact with skin, clothing or surfaces. And internal contamination can occur when this material is inhaled, ingested, or even when it comes into contact with the bloodstream through wounds.
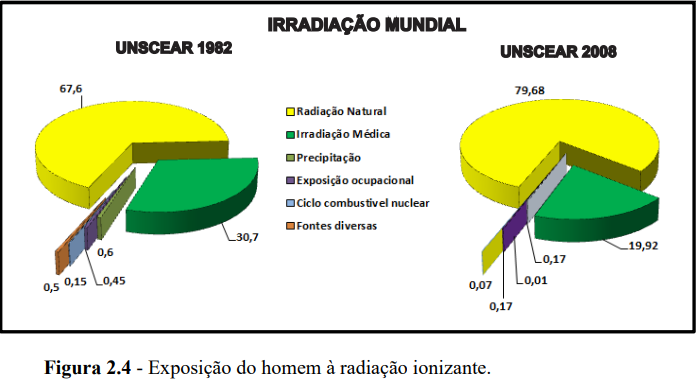
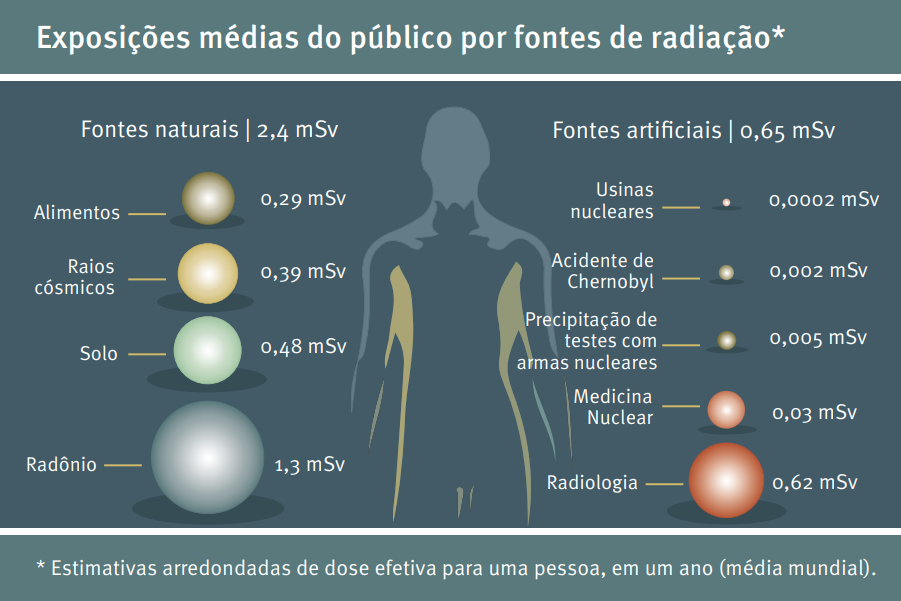
Nowadays, we are constantly exposed to radiation, as it is present in various media, with approximately 80% of radiation exposure coming from natural sources and around 20% from what are considered artificial sources.
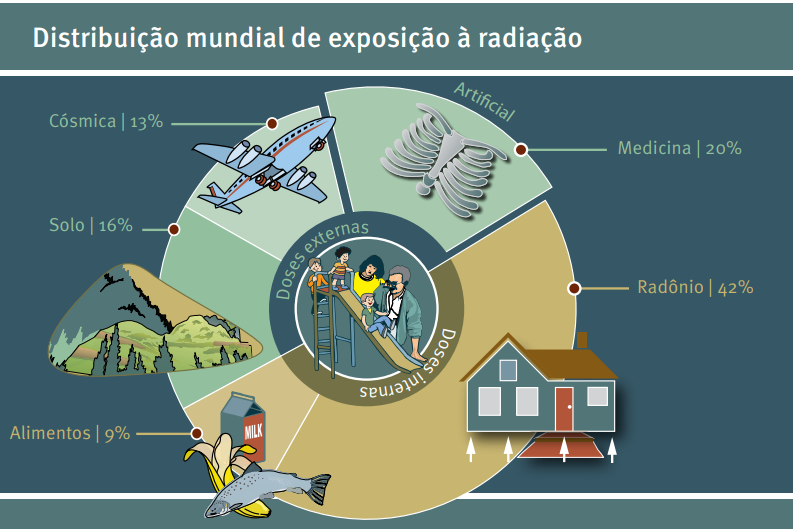
Sources of natural radiation are soil, water/food, radon and cosmic radiation (from various regions of space). Artificial radiation, on the other hand, comes from uses in branches such as: military (as in the use of nuclear weapons); medical (in the use of diagnostic imaging and treatment of certain diseases with controlled doses of radiation); and domestic (as in the case of smoke detectors).
There are various parameters that define water quality, including chemical, microbiological and physical, in order to guarantee the protection of human health. Therefore, water used for human consumption can contain radionuclides from natural or artificial sources that are capable of causing risks to human health and which, depending on the dose and exposure, imply significant risks.
When there is exposure to high doses of radiation in a short space of time, there is a reaction called Acute Radiation Syndrome, in which the exposed individual has various symptoms such as nausea and vomiting, fever and dehydration, which can lead to death. However, damage to the genetic material (DNA) present in cells, causing tumors, leukemia, damage to reproductive cells and genetic disorders occurs when there is long-term exposure to radiation.
The global average effective dose (dose capable of causing damage to different organs and tissues) per year is 2.4 mSv per person, the vast majority of which is exposure to radiation from natural sources, and may vary depending on where these people live. Through the Basic Guidelines for Radiological Protection CNEN NN 3.01, the National Nuclear Energy Commission (CNEN) determined the recommended doses to be applied in Brazil.
In 1977, the first legislation for monitoring and controlling water for human consumption was introduced in Brazil, followed by other ordinances that established the reference limit value for total alpha and total beta radioactivity parameters, which were 0.1 Bq/L for Total Alpha and 1.0 Bq/L for Total Beta.
Until 2010, when the legislation in force for drinking water was MS Ordinance No. 518 of March 25, 2004, the limits remained those mentioned above. If the values were exceeded, the radionuclides (chemical species that emit radiation) had to be identified and quantified and their values compared with the limits set by the National Nuclear Energy Commission (CNEN).
In December 2011, Ordinance No. 2,914 was enacted, changing the reference limit value for drinking water to 0.5 Bq/L for Total Alpha and 1.0 Bq/L for Total Beta. If the limits were exceeded, the concentration of specific radionuclides had to be determined, namely Radium-226 and Radium-228.
In 2021, Ministerial Order GM/MS 888 was published, with new procedures for quantifying Alpha Total and Beta Total in drinking water. There were no changes to the reference limit value, but there were some changes to the procedures for exceeding the limit. Thus, if the Total Beta limit is exceeded, the levels of the radionuclide K-40 (Isotope of the potassium atom with mass 40u) must be quantified and its value deducted from the total value of Total Beta radioactivity. If the value remains above the limit, another sample should be taken and analyzed, and if the values are still above the limit, CNEN REGULATORY POSITION 3.01/012:2020 should be consulted to take action. From then on, CNEN is responsible for analyzing the radiological potability of the water.
Legislation is evolving in line with available studies on Alpha and Beta in drinking water and their toxicity to humans and the environment, ensuring that monitoring is carried out in accordance with current legislation. This is evident when we look at the evolution of drinking water legislation in relation to radiological levels in recent years. There has been considerable progress since 2004.
EP Analítica is constantly striving to evolve its testing services. With this in mind, the technical team has prepared itself and is now fully capable of providing environmental analysis services for Total Alpha and Total Beta Radioactivity.
We have state-of-the-art equipment and fully up-to-date methods for carrying out this analysis and complying with the limits expressed in current legislation.
Article by Catharina Mariusso and Leonardo Paiva
Bibliography
Ministry of Health. Available at: <https://bvsms.saude.gov.br/bvs/saudelegis/gm/2021/prt0888_07_05_2021.html>. Accessed on: 23 Dec. 2024.
Available at: <https://www.if.ufrgs.br/tex/fis01001/radio.pdf>. Accessed on: 23 Dec. 2024.
RADIATION – EFFECTS AND SOURCES. Available at: <https://www.aben.com.br/Arquivos/544/544.pdf>. Accessed on: 23 Dec. 2024.
Available at: <https://www.ufrgs.br/colegiodeaplicacao/wp-content/uploads/2020/06/RADIOATIVIDADE-%E2%80%93-2%C2%AA-PARTE1_removed.pdf>. Accessed on: 20 Dec. 2024a.
RESEARCH AND REFERENCE LEVELS FOR RADIOACTIVITY IN DRINKING WATER. Available at: <https://www.gov.br/cnen/pt-br/acesso-rapido/normas/grupo-3/grupo3-pr301_12.pdf>. Accessed on: 26 Dec. 2024.
Available at: <https://www.gov.br/cnen/pt-br/acesso-rapido/normas/grupo-3/grupo3-nrm301.pdf>. Accessed on: 26 Dec. 2024c.
Available at: <https://www.gov.br/ird/pt-br/central-de-conteudo/publicacoes/RadioatividadeemAguaPotavel.pdf>. Accessed on: 26 Dec. 2024d.
Radiation. Available at: <https://fiocruz.br/biosseguranca/Bis/virtual%20tour/hipertextos/up1/radiacao.html>. Accessed on: 26 Dec. 2024.
BUSHBERG, J. T. Exposure and radioactive contamination. Available at: <https://www.msdmanuals.com/pt/profissional/les%C3%B5es-intoxica%C3%A7%C3%A3o/exposi%C3%A7%C3%A3o-e-contamina%C3%A7%C3%A3o-radioativa/exposi%C3%A7%C3%A3o-e-contamina%C3%A7%C3%A3o-radioativa>. Accessed on: 26 Dec. 2024.
Ministry of Health. Available at: <https://bvsms.saude.gov.br/bvs/saudelegis/gm/2011/prt2914_12_12_2011.html>. Accessed on: 26 Dec. 2024b.
D79367. Available at: <https://www.planalto.gov.br/ccivil_03/decreto/1970-1979/d79367.htm>. Accessed on: 26 Dec. 2024.
